“`html
Complete Guide to How to Find Molar Mass
Molar mass is a fundamental concept in chemistry that plays a crucial role in various scientific calculations, from determining the composition of compounds to understanding chemical reactions. This comprehensive guide will walk you through practical steps to find molar mass and provide essential strategies for mastering the subject in 2025.

Understanding Molar Mass
The **definition of molar mass** refers to the mass of one mole of a substance, usually measured in grams per mole (g/mol). It is highly significant in chemistry as it allows scientists to convert between mass and moles in chemical reactions. Knowing the **average molar mass** helps in preparing solutions and carrying out stoichiometric calculations that involve **measurements of substances**. Furthermore, different compounds can have vastly different molar masses, which can significantly affect their chemical behavior.
Why Molar Mass is Important
The importance of **molar mass** emerges from its implications in both theoretical and applied chemistry. For instance, when doing reaction calculations, knowing the masses of reactants and products is essential for predicting yields and understanding the progression of chemical reactions. Additionally, incorrect molar mass calculations can lead to inaccurate results, affecting the outcomes of experiments in laboratories when analyzing compounds or conducting synthesis reactions.
Units of Molar Mass
Molar mass is typically expressed in **molar mass units** or grams per mole (g/mol). This unit serves as a bridge between the molecular weight of a substance (derived from the mass of its constituent atoms) and the quantity of that substance in moles. Understanding this unit and comfortably converting between moles and mass are key components of stoichiometry in chemistry. Accurately calculating the **molar mass of elements** and compounds ensures precise stoichiometric relationships during chemical reactions.
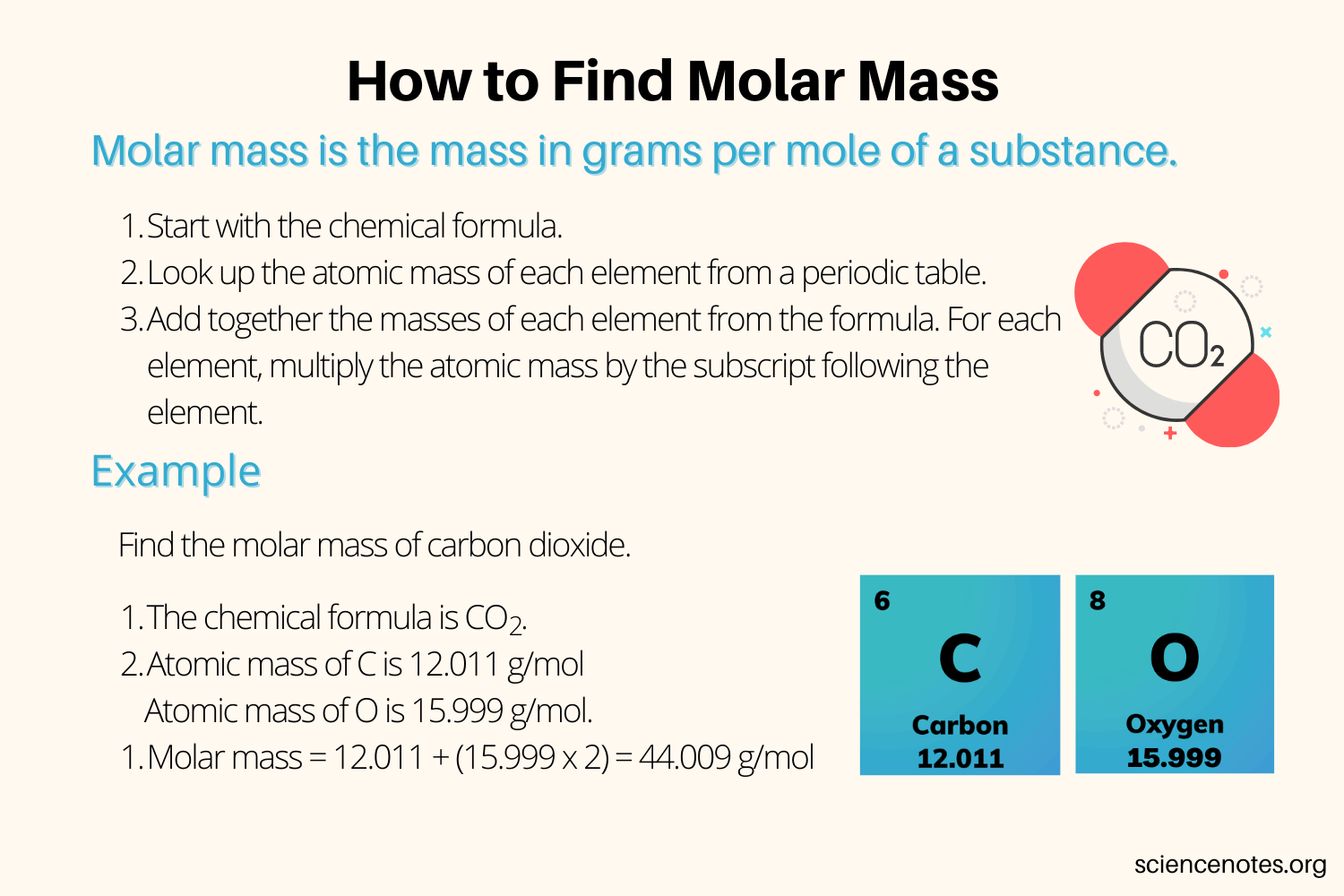
How to Calculate Molar Mass
To **calculate molar mass**, follow these straightforward steps: First, identify the chemical formula of the substance whose molar mass you need to determine. Then, use the **molar mass periodic table** to find the atomic masses of each element in the formula.
Using the Periodic Table for Molar Mass
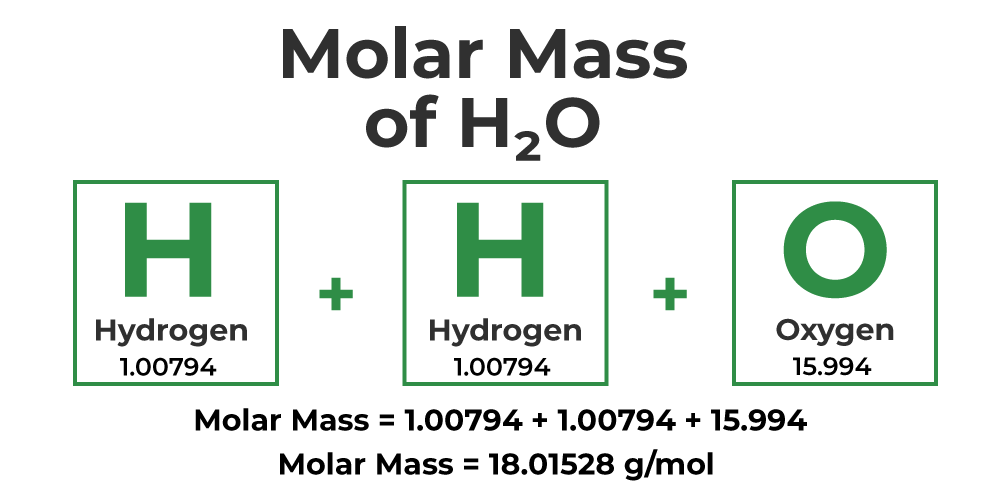
The **molar mass periodic table** is an invaluable resource when determining molar mass. For example, consider water (H2O). To find its molar mass, refer to the periodic table for hydrogen (H, atomic mass = 1.01 g/mol) and oxygen (O, atomic mass = 16.00 g/mol). Water’s formula signifies two hydrogen atoms and one oxygen atom, leading to the calculation: (2 x 1.01) + (1 x 16.00) = 18.02 g/mol. This result represents the **water molar mass**.
Molar Mass Calculations for Compounds
Calculating molar mass for compounds, whether simple or complex, follows the same principle. For a compound like calcium carbonate (CaCO3), identify the atomic masses from the periodic table: calcium (Ca = 40.08 g/mol), carbon (C = 12.01 g/mol), and oxygen (O = 16.00 g/mol). Using the formula that signifies one calcium atom, one carbon atom, and three oxygen atoms results in the molar mass calculation of CaCO3: (1 x 40.08) + (1 x 12.01) + (3 x 16.00) = 100.09 g/mol. Thus, understanding how to calculate molar mass is crucial for accurate chemical assessments.
Practical Examples of Molar Mass
Offering practical examples of **molar mass calculations** can enhance understanding. A common scenario involves finding the **molar mass of ionic compounds** and **organic compounds**. Using **empirical formulas** can simplify tasks that require compounds to be calculated step-by-step.
Example of Molar Mass for Ionic Compounds
To find the **molar mass of sodium chloride (NaCl)**, start by identifying the constituent elements from the periodic table: sodium (Na = 22.99 g/mol) and chlorine (Cl = 35.45 g/mol). Thus, the calculation becomes: (1 x 22.99) + (1 x 35.45) = 58.44 g/mol. Knowing how to compute this value is crucial for its applications in various chemical reactions.
Molar Mass in Organic Chemistry
In organic chemistry, let’s examine glucose (C6H12O6). The calculation involves identifying carbon’s atomic mass (C = 12.01 g/mol), and therefore we compute the molar mass of glucose: (6 x 12.01) + (12 x 1.01) + (6 x 16.00) = 180.18 g/mol. This is an example to demonstrate understanding of how complex organic molecules can be assessed using their **molecular formulas**.
Molar Mass and Properties
The relationship between **molar mass** and other chemical properties introduces significant insight for scientists. Notably, molar mass has implications in volumetric calculations, density considerations, and molarity of solutions.
Relationship Between Molar Mass and Density
Density is fundamentally related to **molar mass**. The molar mass of gases can be predicted by understanding gas laws and molar volumes under various conditions. Essentially, substances with lower molar mass will usually display higher velocities while gases with higher molar masses slow down. Through discrepancies in molar mass or when calculating molar mass for gases, adjusting for temperature and pressure becomes crucial to avoid erroneous results.
Molarity and Molar Mass in Solutions
Utilizing **molarity and molar mass** can streamline solution chemistry processes. Molarity, defined as the moles of solute per liter of solution, can further be enhanced by understanding how to calculate the necessary mass of a solute required for creating molar solutions. For example, to create 1 L of a 1 M NaCl solution, calculate the molar mass of NaCl (as done previously) and weigh out 58.44 grams of NaCl for dissolution in water, ensuring that the total volume remains at 1 L. This hands-on application highlights the significance of **molar mass in chemistry**.
Key Takeaways
- Molar mass is essential in converting between moles and grams in chemical calculations.
- Utilizing the periodic table is critical for accurate molar mass calculations of structures.
- Practical examples enhance comprehension in both organic and inorganic compounds.
- Understanding the relationship between molar mass and properties like density aids in more in-depth chemical analyses.
- Effective applications of molar mass facilitate various laboratory techniques and solutions.
FAQ
1. What is the significance of molar mass in chemical reactions?
The significance of molar mass in chemical reactions lies in its ability to help calculate the amounts of reactants and products in stoichiometry. Accurate molar mass knowledge allows scientists to predict reaction yields and balance chemical equations effectively.
2. How can discrepancies in molar mass affect scientific experiments?
Discrepancies in molar mass can lead to inaccuracies in experimental results, affecting both the potential yields and the kinetics of reactions. Such inaccuracies may derive from relying on outdated or incorrect values from periodic tables.
3. What is the relationship between molar mass and density?
The relationship between molar mass and density is essential, especially in gas laws; substances with higher molar mass typically display lower gaseous velocities while density acts as a guiding metric in solution preparation and reaction forecasts.
4. How do I measure molar mass in a lab setting?
Measuring molar mass in a lab involves using techniques such as titration for solutions or gravimetry. This often includes weighing samples to develop an empirical formula leading to the precise molar mass.
5. What typical values can be expected for common molar masses?
Typical molar masses vary greatly depending on the substance. For example, common values include water at 18.02 g/mol, carbon dioxide at 44.01 g/mol, and glucose at 180.18 g/mol. Understanding these values enables easier comparisons in chemical reactions.
“`